Study helps explain why tuberculosis vaccines are ineffective
Designing effective TB vaccine may require shift in strategy
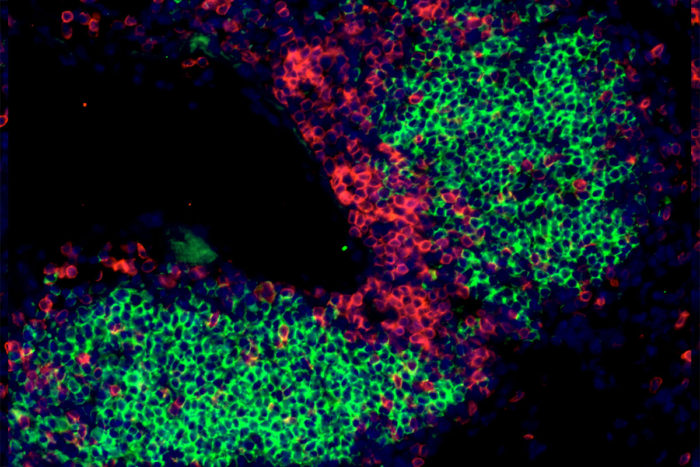 Javier Rangel-Moreno
Javier Rangel-Moreno Many people infected with the bacteria that causes tuberculosis – including people who have been vaccinated – can’t eliminate the bacteria and instead wall them off into protective balls in their lungs, shown above in green. New research suggests TB vaccines are only partially protective because they elicit an immune response that is too slow, suggesting that a successful vaccine would need to produce a faster immune response.
For decades, scientists have worked diligently to develop a more effective vaccine against tuberculosis, but their efforts have been only partially successful – and new research explains why.
Tuberculosis is the leading cause of death from infectious disease worldwide. About 2 billion people are infected with the bacteria that causes tuberculosis (TB), and just under 2 million die from it every year. There is a vaccine for TB, but it is nearly a century old and while it provides good protection against the more severe forms of the disease in children, it is less effective in young adults. Still, it is widely used because no better options exist.
A new study, published Dec. 22 in Nature Communications, helps explain why development of a better vaccine has been stymied. The findings suggest that the current TB vaccine and investigational vaccine candidates elicit immune responses that fail to control the infection not because the responses are too weak, but because they are too slow. In people vaccinated against TB and later infected with the bacteria, activation of immune cells is delayed, allowing the bacteria to multiply.

“It’s not a question of the magnitude of the immune response, it’s the timing,” said Shabaana Abdul Khader, PhD, an associate professor of molecular microbiology at Washington University School of Medicine in St. Louis and the study’s senior author. “Many people in the field of TB vaccine development have been working on increasing the strength of the immune response, and we could go on doing that, but if the timing is the same as for every other vaccine, it’s not going to change the outcome.”
The TB vaccine used around the world, known as Bacillus Calmette–Guérin (BCG), reduces the chance of infection by 20 percent. A truly effective vaccine, like the one for measles, reduces infection by 95 percent or more.
“The first modern TB vaccine recently was tested in people, and it did no better at preventing disease than BCG,” Khader said. “We know that it elicits a strong immune response, but somehow that didn’t translate into protection.”
The idea behind vaccination is that giving the immune system a preview of an infectious microbe allows it to rapidly swing into action when it later encounters that same microbe. Ideally, vaccinated people can mount an immune response within a few days and head off the infection before it even makes them sick.
“We have a dozen or so vaccine candidates in the pipeline, but the problem is that they don’t reduce the bacterial load significantly enough in animal models, even though they elicit strong immune responses in the laboratory,” Khader said. “So we asked ourselves, ‘If all the vaccines we are developing are not working very effectively, why aren’t they working?’”
Previous researchers had noted that vaccination – either with BCG or investigational vaccine candidates – doesn’t speed up the immune response against TB much. Even in vaccinated mice, activated T cells – a kind of immune cell crucial for controlling the infection – don’t start arriving at the site of infection for about two weeks, giving the bacteria time to multiply to high levels.
To find out whether speeding up the immune response would make an ineffective vaccine effective, Khader, postdoctoral researcher Kristin Griffiths, PhD, and colleagues tried a new approach. They vaccinated mice with BCG, boosted a month later, and a month after that, challenged the mice with Mycobacterium tuberculosis, the bacteria that causes TB, while also giving the mice immune cells specially prepared to activate T cells.
With these extra immune cells jump-starting the immune response, the T cells arrived, activated and ready to fight, seven to eight days after infection, rather than 12 to 14 days. The number of bacteria in the mice’s lungs, rather than dropping by a factor of 10 or 100, dropped to nearly undetectable levels.
The researchers repeated the experiment with a different TB vaccine. The results were the same: The immune response started earlier, and the bacteria were all but eliminated.
This study is a proof of concept that shows that the timing, rather than the strength, of the immune response determines whether a TB vaccine is effective. The problem is that the technique Khader and colleagues used to speed up the response – administering extra immune cells at the time of TB infection – cannot be replicated in real life, because there is no way of knowing when people will be exposed to the bacteria.
“In a way, this is really disappointing,” Khader said. “We start thinking that maybe none of the vaccines we have for TB will work. But then we come back to the table and say, ‘Let’s put our disappointment aside and figure out what we can really do from here.’ We might have to design an entirely different kind of vaccine if we want to elicit an immune response that eradicates infection. Or maybe eradicating infection isn’t a realistic goal, but we can still make a vaccine that prevents disease or delays TB reactivation. We’re looking at vaccines for TB from a different point of view now, and that’s exciting.”







