Scientists unravel mysteries of cells’ whiplike extensions
Describing the structure of cilia opens doors to understanding range of diseases
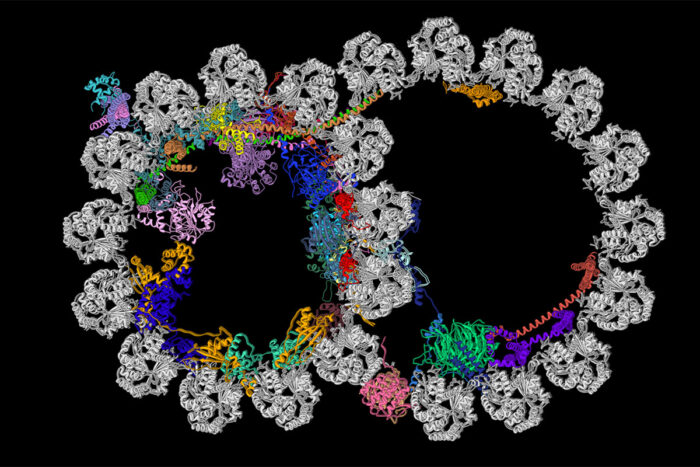 R. Zhang, A. Brown
R. Zhang, A. BrownCilia contain structures called ciliary doublet microtubules. Pictured is a cross section of one of these structures. A new study from Washington University School of Medicine in St. Louis and Harvard Medical School has described the most detailed picture yet of these vital cellular structures. The microtubule is shown in gray, and the newly identified proteins decorating the microtubule are depicted in various colors.
Cilia, or flagella — whiplike appendages on cells — perform diverse tasks required to keep the body healthy. When cilia malfunction, the consequences can be devastating, causing a range of problems, from blindness, to lung and kidney diseases, to congenital heart defects. Now, scientists have revealed the first detailed look at the inner structure of cilia.
The newly revealed structure offers a starting point to begin exploring how cilia are assembled during development, how they are maintained over a cell’s life span, and how they might become dysfunctional if some of the cogs in these complex molecular machines are mutated or missing. The structure of these microscopic molecular machines common to cells in organisms from algae to people potentially will answer questions about human health and disease.
The research, by investigators at Washington University School of Medicine in St. Louis and Harvard Medical School, was published recently in the journal Cell.
“This new study is exciting because it fills in a lot of missing information about the structure of cilia,” said senior author Rui Zhang, PhD, an assistant professor of biochemistry and molecular biophysics at Washington University. “When cilia don’t work properly, bad things happen. We need to know details of the structure in order to develop treatments for diseases, or strategies to prevent the developmental defects that can occur in the early embryo if the cilia are not functioning as they should.”
In the respiratory tract, cilia move mucus and protect against viral and bacterial illnesses. In the reproductive tract, they propel sperm to fertilize an egg. Cilia also perform vital tasks in the brain, the kidney, the pancreas and in bone growth. And in the earliest stages of development, the rotational motion of specialized cilia in the embryo defines the body’s left-right asymmetry and where organs are placed. Without properly functioning cilia, the heart may not end up on the left side, where it should be, and it may not function properly.
Cilia are implicated in multiple human disorders, including polycystic kidney disease, which affects some 600,000 Americans and requires dialysis; primary ciliary dyskinesia, which causes chronic lung disease, misplaced organs and infertility; Bardet-Biedl syndrome, which causes patients to become blind in childhood and leads to diabetes, kidney disease and extreme obesity; and many congenital heart defects, which occur when left-right asymmetry goes awry and require complex surgeries to repair.
In the new study, the researchers used a technique called single particle cryo-electron microscopy to get a first look at 33 specific proteins arranged inside cilia — within structures called ciliary microtubule doublets — in a strict repeating pattern.
“Before this work, everyone assumed these proteins inside cilia just stabilize the structure, which is true for a subset of the proteins, especially when you consider the forces produced by the continuous beating of the cilia,” Zhang said. “But based on how they are arranged inside this structure, we believe these proteins are doing many more things.”
Since many of the proteins protrude through the cilia, Zhang and his colleagues speculate that they may allow for communication between the inside and the outside of the ciliary microtubule doublets; govern the function of enzymes that make important biochemical reactions possible; and sense changes in the calcium concentration of the environment, which plays a role in triggering the cilia to beat.
“Among the proteins identified, five are associated with diseases that have been studied in mice and people,” said co-author Susan K. Dutcher, PhD, a professor of genetics at Washington University. “But until now, no one knew that these proteins were found inside cilia. We are just beginning to understand their roles in normal and disease states.”
The researchers studied cilia in a type of algae called Chlamydomonas reinhardtii, which are single-celled organisms that have cilia structurally and biochemically similar to those of more complex organisms, including people. One question Dutcher is interested in answering is how the proteins making up cilia structure govern the type of motion that the cilia perform. The cilia of single-celled C. reinhardtii are capable of more than one type of motion.
“In some situations, the cilia are doing what you might consider a breast stroke,” Dutcher said. “In others, the motion is more of an S-shaped wave. The cilia of many cells in mammals can only produce one of these motions. But the single-celled C. reinhardtii, perhaps to help it adapt to its environment, can switch between them. That’s why we’re studying algae at a medical school — the genetic problems we can study in the cilia of these organisms are similar to the ones that can occur in people, often with devastating consequences.”
Zhang, Dutcher and their colleagues have plans to use the latest techniques of cryo-electron microscopy to study the Chlamydomonas mutants of each of the 33 proteins inside cilia to seek answers to many questions that have arisen from this new and detailed knowledge of the structure.







