Research offers clues for treating fatal neurological disorder in kids
Enzyme replacement therapy, in mice and sheep, slowed brain degeneration
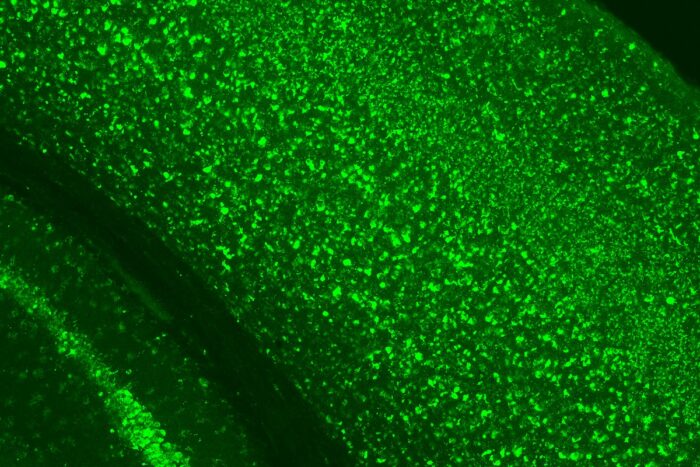 Hemanth Ramesh Nelvagal
Hemanth Ramesh NelvagalIn this slide, bright green staining shows the massive buildup of material that occurs inside brain cells when their lysosomes, the cells’ waste disposal and recycling system, are defective. This occurs in CLN1 disease, a fatal neurological disorder that affects infants and young children, because a vital lysosomal enzyme is missing. Research in animals led by Washington University in St. Louis and the Roslin Institute in Scotland shows that supplying this missing enzyme helps improve the condition.
At birth, the children appear healthy. But within a few years, toddlers and young children with infantile Batten disease, a rare but fatal brain disorder, succumb to blindness, seizures, dementia and become unable to walk. No cure exists, and most die in early childhood.
But new research in animals by scientists at Washington University School of Medicine in St. Louis and the Roslin Institute at the University of Edinburgh in Scotland suggests enzyme replacement therapy may slow brain degeneration. The Washington University researchers evaluated the therapy in mice, and researchers in Scotland evaluated the treatment in a sheep model of the disease. The findings underscore potential treatments for the genetic condition, also known as CLN1 disease.
The study was published recently in the Journal of Clinical Investigation.
“Our work has shown the potential for a new therapy to treat this devastating fatal disease,” said the study’s co-senior author, Jonathan D. Cooper, PhD, a professor of pediatrics, of genetics and of neurology at Washington University. “Not only did we improve the disease in mice, but we were successful in scaling it up to have similar partial efficacy in the much larger brain of a sheep model of the same disease. Our goal is to be able to treat children with Batten disease, and this is an important step forward.”
Batten disease refers to a group of inherited nervous system disorders that involve a cell’s inability to remove and recycle cellular waste. The condition, also known as neuronal ceroid lipofuscinoses, is named after the waste that builds up inside cells. This disease often begins in childhood, and is classified by the culprit gene and age of symptom onset, which varies according to which gene is mutated.
The number of people who have Batten disease remains unknown; however, some researchers have estimated it affects two to four of every 100,000 children in the U.S.
“Children with this disease lack one very important enzyme that normally helps break down material inside cells so that it can be recycled,” Cooper said. “When the enzyme is missing, this causes progressive brain degeneration that leads to death.”
Using genetically modified mice and sheep, the scientists found that supplying the animals’ brains and spinal cords with monthly doses of the deficient enzyme, known as PPT1, reduced the severity of the disease. Specifically, the infusions improved motor function, diminished signs of disease within the brain and, over a six-month period, decreased brain matter loss.
“Using mice, we were able to deliver an appropriate dose of enzyme and determine the best route for delivery,” Cooper explained. “We were then able to extrapolate this method and scale it up to have similar benefits in the much larger sheep brain. This is a critical step for whether we could ultimately do the same for affected children.”
Scientists at the Roslin Institute — famous for cloning Dolly the sheep in 1996 — developed a sheep model for infantile Batten disease using the genome editing technology CRISPR/Cas9. “Mice have relatively simple brains, but because sheep brains are closer in size and complexity to human children, they are ideal in finding out if we could also treat a much larger brain,” Cooper said. “Sheep with the missing enzyme display symptoms similar to children with the disorder, including changes in brain size. Our colleagues in Scotland measured these with MRI imaging much like you would do in children, and we showed corresponding improvements in brain pathology.”
Added Tom Wishart, PhD, a professor of molecular anatomy and deputy director of the Roslin Institute: “This study could only have been done by a collaborative research team. Such work is a key step toward everyone’s ultimate goal of safely carrying out clinical tests of potential treatments in children affected by this devastating condition. Through studies in sheep, we gain invaluable insights into the progression of this condition, which can guide our work toward developing an effective therapy for affected children.”







