Rare form of diabetes may require alternate treatment
Current therapies linked to destruction of insulin-secreting cells that regulate blood sugar
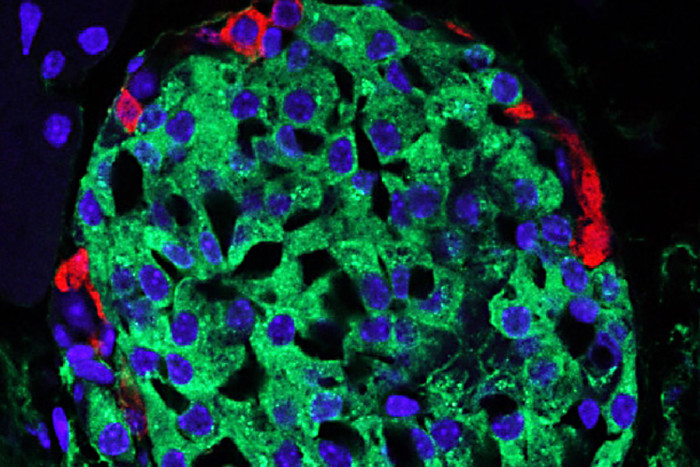 Mills laboratory
Mills laboratoryIn the pancreas, the beta cells (green, with blue nucleus) secrete insulin, but in the genetic form of diabetes called MODY1, those cells are under stress. Standard therapies are designed to make the beta cells secrete more insulin, but new research indicates that the drugs also increase stress levels in the cells, eventually killing beta cells and requiring patients to inject insulin.

Patients with a rare, genetic form of diabetes often are misdiagnosed as having type 2 diabetes because the two share symptoms.
But new research at Washington University School of Medicine in St. Louis suggests that treating such patients with therapies designed for type 2 diabetes is potentially harmful and that treatment guidelines need to change. The underlying problems in patients with the genetic form of the disease — called maturity-onset diabetes of the young (MODY1) — are very different from those in type 2 diabetes. And treating MODY1 patients with drugs for type 2 diabetes appears to lead to destruction of insulin-secreting beta cells that regulate blood sugar, the scientists found.
The findings are published March 18 in The Journal of Biological Chemistry.
“People diagnosed with type 2 diabetes are treated with oral medications that make insulin-secreting beta cells very active,” said first author Benjamin D. Moore, PhD, a former postdoctoral fellow at Washington University who is now at Massachusetts General Hospital. “But the MODY1 pathway we’ve uncovered shows that stimulating those cells with those drugs can lead to beta cell death. That means these patients can become dependent on insulin injections much sooner.”
Moore said it’s common for patients with MODY1— who make up 3 to 5 percent of all patients with diabetes — to transition from oral medications to insulin injections within 10 years of diagnosis as a way to keep their blood sugar in check. The new research suggests, however, that revving up the beta cells with oral medications increases cellular stress levels and eventually kills those cells, thus precipitating the need to switch to insulin.
Instead, patients with MODY1 might benefit from therapies that target a specific pathway that appears to be essential to the function of insulin-secreting cells. The researchers noted a relationship between a pair of proteins that regulate cells that secrete insulin and enzymes in the stomach, liver, kidney and intestines. One of those proteins is made by the gene that’s altered in patients who have MODY1 diabetes.
Working with Jason C. Mills, MD, PhD, an associate professor of medicine at Washington University, Moore originally was studying cells in the stomach when he identified the insulin-secretion pathway disrupted in the genetic form of diabetes.
Mills and Moore believe the pathway they’ve identified in MODY1 also may be important in disorders that involve cells that secrete other enzymes.
“Nature doesn’t re-invent the wheel,” Mills said. “What these different cells secrete is very different, but the machinery is very similar. As with auto plants, although a BMW is very different from a Volkswagen, the factories where those cars are built are not really that different. It appears the same thing may be true for a number of these cells that secrete key enzymes in the gastrointestinal tract.”
Mills’ laboratory is continuing to conduct experiments to determine whether the same pathways are active in different types of secretory cells. Meanwhile, they believe doctors treating patients with diabetes need to determine whether they have the MODY1 form of the disorder before moving ahead with treatment.
“It’s important to diagnose patients as accurately as possible and to attempt to target the correct pathway,” Moore said.







