New imaging technique aims to ensure surgeons completely remove cancer
Combines light, sound to create detailed images of cells more quickly than current approach
 Terence T. W. Wong
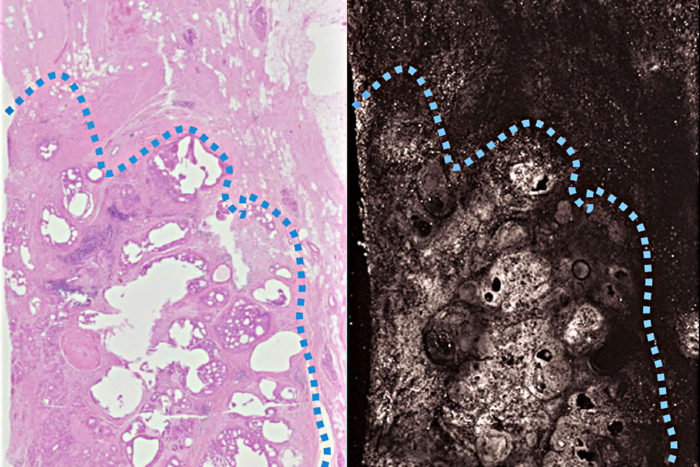
Terence T. W. WongA new imaging technique based on light and sound produces images doctors can use to distinguish cancerous breast tissue (below the dotted blue line) from normal tissue more quickly than is currently possible. Pathologists routinely inspect surgical specimens to make sure all cancerous tissue has been removed. The new technique (right) produces images as detailed and accurate as traditional methods (left), but in far less time. The researchers are working to make the technique fast enough to be used during a surgery, so patients don't have to return for a second surgery.
Of the quarter-million women diagnosed with breast cancer every year in the United States, about 180,000 undergo surgery to remove the cancerous tissue while preserving as much healthy breast tissue as possible.
However, there’s no accurate method to tell during surgery whether all of the cancerous tissue has been successfully removed. The gold-standard analysis takes a day or more, much too long for a surgeon to wait before wrapping up an operation. As a result, about a quarter of women who undergo lumpectomies receive word later that they will need a second surgery because a portion of the tumor was left behind.
Now, researchers at Washington University School of Medicine in St. Louis and California Institute of Technology report that they have developed a technology to scan a tumor sample and produce images detailed and accurate enough to be used to check whether a tumor has been completely removed.
Called photoacoustic imaging, the new technology takes less time than standard analysis techniques. But more work is needed before it is fast enough to be used during an operation.
The research is published May 17 in Science Advances.
“This is a proof of concept that we can use photoacoustic imaging on breast tissue and get images that look similar to traditional staining methods without any sort of tissue processing,” said Deborah Novack, MD, PhD, an associate professor of medicine, and of pathology and immunology, and a co-senior author on the study.
The researchers are working on improvements that they expect will bring the time needed to scan a specimen down to 10 minutes, fast enough to be used during an operation. The current gold-standard method of analysis, which is based on preserving the tissue and then staining it to make the cells easier to see, hasn’t gotten any faster since it was first developed in the mid-20th century.
For solid tumors in most parts of the body, doctors use a technique known as a frozen section to do a quick check of the excised lump during the surgery. They look for a thin rim of normal cells around the tumor. Malignant cells at the margins suggest the surgeon missed some of the tumor, increasing the chances that the disease will recur.
But frozen sections don’t work well on fatty specimens like those from the breast, so the surgeon must finish a breast lumpectomy without knowing for sure how successful it was.
“Right now, we don’t have a good method to assess margins during breast cancer surgeries,” said Rebecca Aft, MD, PhD, a professor of surgery and a co-senior author on the study. Aft, a breast cancer surgeon, treats patients at Siteman Cancer Center at Barnes-Jewish Hospital and Washington University School of Medicine.
Currently, after surgery a specimen is sent to a pathologist, who slices it, stains it and inspects the margins for malignant cells under a microscope. Results are sent back to the surgeon within a few days.
To speed up the process, the researchers took advantage of a phenomenon known as the photoacoustic effect. When a beam of light of the right wavelength hits a molecule, some of the energy is absorbed and then released as sound in the ultrasound range. These sound waves can be detected and used to create an image.
“All molecules absorb light at some wavelength,” said co-senior author Lihong Wang, PhD, who conducted the work when he was a professor of biomedical engineering at Washington University’s School of Engineering & Applied Science. He is now at Caltech. “This is what makes photoacoustic imaging so powerful. Essentially, you can see any molecule, provided you have the ability to produce light of any wavelength. None of the other imaging technologies can do that. Ultrasound will not do that. X-rays will not do that. Light is the only tool that allows us to provide biochemical information.”
The researchers tested their technique by scanning slices of tumors removed from three breast cancer patients. For comparison, they also stained each specimen according to standard procedures.
The photoacoustic image matched the stained samples in all key features. The architecture of the tissue and subcellular detail such as the size of nuclei were clearly visible.
“It’s the pattern of cells – their growth pattern, their size, their relationship to one another – that tells us if this is normal tissue or something malignant,” Novack said. “Overall, the photoacoustic images had a lot of the same features that we see with standard staining, which means we can use the same criteria to interpret the photoacoustic imaging. We don’t have to come up with new criteria.”
Having established that photoacoustic techniques can produce usable images, the researchers are working on reducing the scanning time.
“We expect to be able to speed up the process,” Wang said. “For this study, we had only a single channel for emitting light. If you have multiple channels, you can scan in parallel and that reduces the imaging time. Another way to speed it up is to fire the laser faster. Each laser pulse gives you one data point. Faster pulsing means faster data collection.”
Aft, Novack and Wang are applying for a grant to build a photoacoustic imaging machine with multiple channels and fast lasers.
“One day we think we’ll be able to take a specimen straight from the patient, plop it into the machine in the operating room and know in minutes whether we’ve gotten all the tumor out or not,” Aft said. “That’s the goal.”







