Morris, Humphreys receive Chan Zuckerberg Initiative funding
Research supports international Human Cell Atlas project
 Haojia Wu
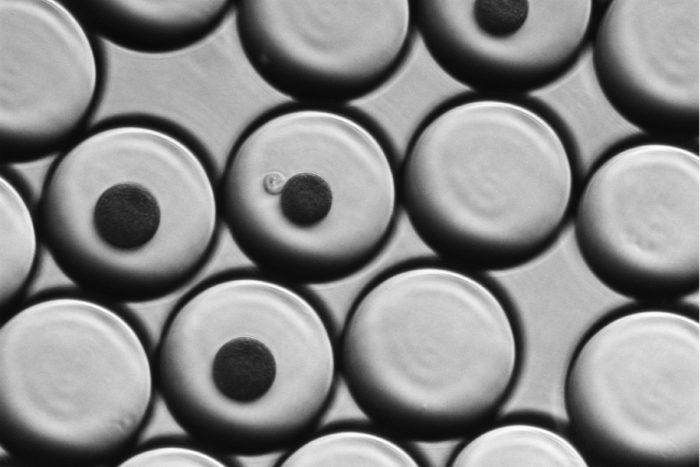
Haojia WuIn single-cell RNA sequencing, individual cells are suspended in droplets of water that also contain a coated bead. When the cell is purposefully broken apart, the coated bead captures the cell's RNA so it can be analyzed. In this image, the large circles are water droplets. Smaller, dark circles are the coated beads. In the center, a single translucent kidney cell from a mouse touches one of the beads. The water droplets are suspended in oil, and each droplet is about the diameter of a human hair.
Two researchers at Washington University School of Medicine in St. Louis have received pilot grants from the Chan Zuckerberg Initiative to support projects that contribute to the Human Cell Atlas, an international effort to create a detailed map of all cells in the human body. Charting the locations of individual cells, unraveling their genetics and revealing how they interact to regulate health can shed light on how things go wrong and result in disease.
The Chan Zuckerberg Initiative was established by Facebook co-founder Mark Zuckerberg and his wife, Priscilla Chan, MD, with the goal of eliminating disease. Part of the effort is aimed at accelerating basic science research as a foundation for developing new ways to fight disease.
Samantha A. Morris, PhD, an assistant professor of developmental biology and of genetics, and Benjamin D. Humphreys, MD, PhD, the Joseph Friedman Associate Professor of Renal Diseases in Medicine, each have received one year of support to conduct what is called single-cell RNA sequencing with a focus on cells in the kidney, liver and small intestine. Work in their labs will map out the relationships between and plumb the biological depths of every one of the millions of individual cells that make up each of those organs.
Single-cell RNA sequencing is a technique that can reveal what genes are turned on in a given cell as well as how high those genes are dialed up or down. Both can influence overall health — or diseases.
“In the past, we needed hundreds of thousands of cells to measure the expression of a handful of genes,” said Humphreys, also director of the Division of Nephrology at the School of Medicine. “Now, we can measure the expression of thousands of genes in a single cell. Single cells are the fundamental unit of life. This kind of resolution allows us to understand what individual cells are doing in a completely unprecedented way.”
Humphreys is a kidney specialist, and one aspect of his research is investigating why some patients with diabetes experience kidney failure quickly while others gradually develop kidney damage. He was searching for better ways to study differences in gene expression between these patients, when, shortly after his arrival at Washington University from Harvard, he attended a lecture given by Morris. She, who also recently had left Harvard, was one of the few School of Medicine investigators focused on single-cell RNA sequencing at the time, Humphreys said.
“Looking at kidney samples under the microscope, we have no way to distinguish which patients will lose kidney function quickly,” he said. “After hearing Samantha talk about this technique, I wanted to use it in my own research. We think the key to unlocking the difference between these two types of patients will be at the single cell level.”
Morris is focused on performing single-cell RNA sequencing on cells in the liver and small intestine. A goal of her research is to engineer a working intestine as a therapy for short bowel syndrome. The condition can lead to malnutrition after a portion of the bowel has been removed, often to treat an intestinal condition such as cancer or Crohn’s disease, and the remaining intestine can’t function properly.
Her research also focuses on developing tools to help standardize the data from single-cell RNA sequencing experiments so that results can be compared across labs. Morris has proposed the development of benchmark cell types that essentially offer a known set of genes that can be run through the equipment to check for accuracy.
“The aim of the Human Cell Atlas is to make a map of every single cell in the human body,” Morris said. “That is going to take a massive international effort. One of the biggest challenges is that we’ll have different labs using different equipment and different techniques to contribute data. We are developing standard cells, with known gene expression, that people can add to their experiments in order to check their work.”







