Landmark Alzheimer’s prevention trial to evaluate third drug
Effort to study drug's ability to prevent, delay the disease
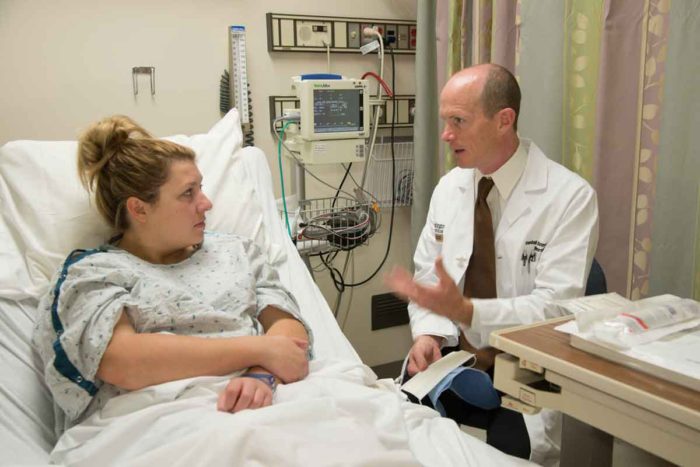 Robert Boston
Robert BostonWashington University School of Medicine's Randall J. Bateman, MD, talks with DIAN-TU trial participant Natalie Shriver, of Omaha, about a study to test drugs that may prevent or delay Alzheimer's disease.
An international team led by Washington University School of Medicine in St. Louis has selected a third investigational drug to be tested in a worldwide clinical trial – already underway – aimed at finding treatments to prevent Alzheimer’s disease.
The third drug is being developed by Janssen Research & Development, LLC, in New Jersey. It is designed to lower production of amyloid beta, a protein that clumps together into plaques and damages neurons in the brain, leading to memory loss, cognitive problems and confusion. The drug is designed to block the enzyme beta secretase — which produces amyloid beta — with a goal of reducing the amount of amyloid beta available to clump and cause neurodegeneration.
This investigational drug joins two others already being evaluated in the Dominantly Inherited Alzheimer’s Network Trial Unit (DIAN-TU) study, which involves people with an inherited predisposition to develop Alzheimer’s at a young age, usually in their 30s, 40s or 50s. Participants already enrolled will continue on their existing drug regimens, and additional volunteers with no or mild symptoms of cognitive impairment will be enrolled to evaluate the third drug.
“We are delighted with the new collaboration with Janssen Research & Development to expand the number of novel therapeutic targets we are testing,” said Washington University Alzheimer’s specialist Randall J. Bateman, MD, director of the DIAN-TU, a public-private-philanthropic research partnership.
“Testing a beta secretase inhibitor in the DIAN-TU trial further diversifies the approach to speed identification of potential preventions and treatments for this devastating disease,” added Bateman, who is also the Charles F. and Joanne Knight Distinguished Professor of Neurology at Washington University.
The DIAN-TU, launched in 2012, is the first trial aimed at identifying drugs to prevent or slow Alzheimer’s in people who are nearly certain to develop the disease due to inherited genetic mutations. Specifically, people in the trial have mutations in one of three genes – APP, PSEN-1 or PSEN-2 – which are linked to early-onset Alzheimer’s. The hope is that by intervening early – before Alzheimer’s ravages the brain – it may be possible to thwart the disease.
As part of the trial, three-quarters of new enrollees will be randomly assigned to receive the beta secretase inhibitor, and one-quarter will receive the placebo. Both groups will be evaluated for at least four years to determine whether the investigational drug delays or prevents the onset of Alzheimer’s disease.
“Janssen welcomes this opportunity for researchers to test the mechanism of beta secretase inhibition in people who have dominantly inherited genetic mutations that put them at substantial risk of early-onset Alzheimer’s disease. The DIAN-TU trials will provide a rigorous and powerful test of the amyloid hypothesis while evaluating a potential preventive treatment option for autosomal dominant Alzheimer’s disease,” said Gary Romano, MD, PhD, the head of Alzheimer’s Disease Development for Janssen Research & Development.
Although the trial focuses on people with rare mutations, treatments that are successful in this population potentially could be used to slow or stop the forms of Alzheimer’s that occur more commonly in older adults. It is thought that the destructive molecular and cellular processes in the brain are much the same for both types of the disease.
The other two investigational drugs already being tested in the DIAN-TU are gantenerumab, an antibody made by Roche that binds to clumps of amyloid beta and helps remove them from the brain, and solanezumab, an antibody made by Eli Lilly and Co. that binds to free-floating fragments of amyloid beta protein, allowing them to be cleared before they clump together to form plaques. Enrollment in these two groups of the trial was completed in 2015, and these participants will be followed through the end of 2019.
Alzheimer’s researchers selected the investigational drugs from more than 20 drugs nominated by pharmaceutical companies. Each drug has a unique approach to counter the toxic effects of amyloid beta. Each also passed earlier clinical trials that evaluated safety and effectiveness of the drugs and whether they targeted amyloid beta in study participants.
“We are pleased to see the DIAN-TU trial researchers continuing to broaden the types of investigational drugs they are testing,” said Maria Carrillo, PhD, chief science officer of the Alzheimer’s Association, which is helping to fund the trial. “Alzheimer’s is a very complex disease, and it is extremely important that we develop therapies to address Alzheimer’s from a variety of angles and at multiple stages of the disease.”
Along with beginning testing of the beta secretase inhibitor, the new arm of the DIAN-TU study uses a new disease progression model to identify changes in cognition earlier and includes more frequent cognitive testing using remote applications.
In addition, the trial will include an investigational imaging-based marker targeting disease progression. This novel radiopharmaceutical tracer – which is being developed by General Electric (GE) Healthcare and called THK-5351 – is designed to detect the brain protein tau by positron emission tomography (PET) scan. Tau accumulates in the brain of individuals with Alzheimer’s, where it forms toxic tangles.
By incorporating a radioactive atom into a molecule that specifically detects tau, researchers may be able to monitor the amount and location of tau tangles in participants’ brains by PET scan. Investigators are hoping to determine whether this imaging method will demonstrate the presence of tau tangles before an individual starts to show symptoms of cognitive decline and if it can help predict the onset of dementia more accurately than existing biomarkers.
The DIAN-TU trial is underway at 24 sites across seven countries. Because of the rarity of dominantly inherited Alzheimer’s disease, the program will be expanded to additional countries, potentially including Argentina, Bulgaria, China, Germany, Japan, Korea, Mexico, Netherlands and Sweden.
For people with Alzheimer’s, family members, doctors and researchers interested in participating, the DIAN-TU launched the DIAN Expanded Registry (DIAN EXR). For more information or to register for potential participation in the trial, go to www.dianexr.org, call 1-844-DIAN-EXR (342-6397) or email dianexr@wustl.edu.







