Is it possible to grow a kidney?
New research is allowing scientists to grow organs like kidneys using embryonic cells from animals such as pigs
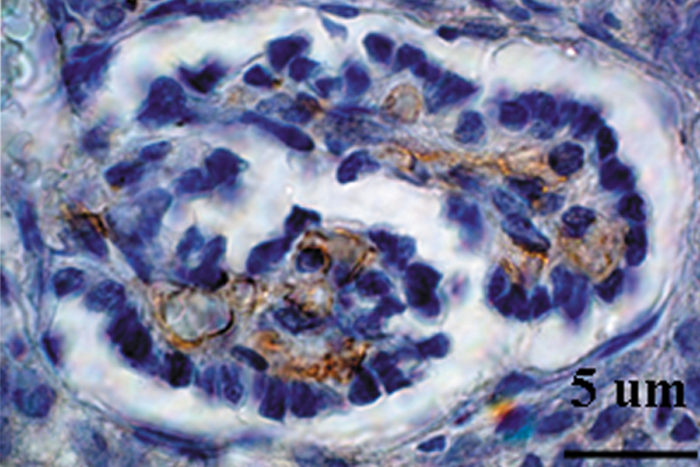
A pig kidney grown in a rat has rat blood vessels (shown in brown).
The simple answer to the question “Is it possible to grow a kidney?” is “Yes,” because Marc Hammerman, MD, and his colleague, Sharon Rogers, MS, have done it—and with greater success than any other researchers in the world.
The more complex answer begins with “Yes,” and then continues by telling about an investigative journey begun in 1996, when Hammerman and Rogers posed the very same question to themselves. Hammerman is the director of the Renal Division at Washington University School of Medicine and Barnes-Jewish Hospital. Rogers is a research instructor in medicine within the division.
“Thomas Edison defined genius as 1 percent inspiration and 99 percent perspiration,” says Hammerman. “Our inspiration came when we hypothesized growing new organs, kidneys and endocrine pancreas using transplanted pig embryonic organ primordia as starting material. We’ve been in the perspiration phase of our research for 17 years now as we’ve worked to prove this can be done.”
Hammerman is a leader in the burgeoning field of organogenesis, which focuses on growing new organs using embryonic cell clusters derived from animals such as the pig. The clusters are known as organ primordia. Unlike stem cells, organ primordia cannot develop into any cell type. Rather, they are locked into becoming a particular cell type or one of a set of cell types that make up an organ.
A complicated organ to grow
“The challenge of growing a kidney is that it is a very complicated organ; you need hundreds of different types of cells for the kidney to work, and they have to come together in exactly the correct configuration,” says Hammerman. “It’s a challenge no one has come close to meeting using embryonic stem cells, because the assembly process is so difficult we can’t figure out how to direct it.”
The genius of Hammerman and Rogers’ technique is that the pig embryonic kidney primordia they transplant into rats already know how to self-assemble. In addition, the researchers have discovered that once transplanted into the host, the primordia attract the blood vessels needed for the organ to function. What makes their patented approach truly unique, however, is their ability to produce a perfect kidney that excretes urine, something other researchers have not accomplished with equal success.

“We achieve this by transplanting not only the embryonic kidney but also the part that differentiates into the ureter. Once that develops, we surgically connect the transplanted ureter with the host’s ureter,” says Hammerman. “All of these procedures are exceedingly painstaking and involve delicate microsurgery of barely visible organs.”
Other research groups have reproduced this process with similar results but have not pursued perfecting the technique. Hammerman believes the reason is that, quite simply, what they are doing is tremendously difficult.
“To come up with the concept of transplanting an embryonic kidney and to think it might become vascularized and differentiate into a perfect kidney—that’s a remarkable idea,” he says. “But at some point, potential has to turn into reality. And that’s hard. Every step of a scientific process that leads to an application in people is difficult. The genesis of an idea is the most difficult step, but it’s more of a creative endeavor. The next steps involve a lot of hard work—solving a large number of problems, some you expect, some surprising, that are necessary just to get the technology to the place where it might be useful as a clinical tool.”
Although the point at which transplanting pig kidney primordia into humans to grow new kidneys is years away, the technique has advanced to the point where new kidneys grown in rats have allowed the animals to live for seven to eight days after the original kidneys were removed.
“The next step will be transplanting pig primordia into primates. We won’t do that, however, until we have devised an immune suppression regimen that permits growth and differentiation of the kidney, and that new kidney will need to keep the animal alive for an extended period of time,” says Hammerman.
Transplanting a pancreas
The move to primates already has been achieved with Hammerman and Rogers’ work transplanting pig embryonic pancreas as a means of treating type 1 and type 2 diabetes in humans.
“When we transplant the pancreas primordia into primates, they differentiate into beta cells, which then multiply in the host,” explains Hammerman. “We can cure diabetes in diabetic rats and improve glucose intolerance in a diabetic primate by quite a bit, although we haven’t been able to completely cure the diabetes long-term; within a month or two, the primate requires insulin. We won’t think about seeking FDA approval to move to human trials until we are able to render a diabetic primate insulin-nonrequiring for at least six months.”
Hammerman never considered himself to be a patient person until beginning his work in organogenesis, but that characteristic and a pioneering spirit are proving to be necessary qualities to working in the field. “Oftentimes when one does science, it’s possible to go to the literature and find someone who has encountered a problem similar to yours and come up with a way to solve it,” says Hammerman. “That’s not the case for us. We are blazing the trail, which means there’s a lot of trial and error. The experiments themselves are time-consuming. It takes 120 days to grow a pig kidney in a rat, because that’s how long it takes in a pig. So four months pass before we know whether or not an experiment has worked.”
Is it worth the time and effort to grow kidneys and pancreas? Hammerman’s answer is an emphatic “Yes.”
“Every year, approximately 10,000 kidneys become available for transplant into patients with end-stage kidney disease,” he says. “But the waiting lists for kidney transplants can run as high as 100,000 individuals. Most patients die of the disease before an organ becomes available. And if we can develop a transplantation cure for both types of diabetes, it would benefit tens of millions of people. I’m convinced therapies based on growing new organs will be part of mainstream medical practice by the middle of the 21st century.”







