International trials underway for childhood malnutrition therapy developed at WashU
Gift from Andy Newman supports world-changing research on the gut microbiome
 Ishita Mostafa
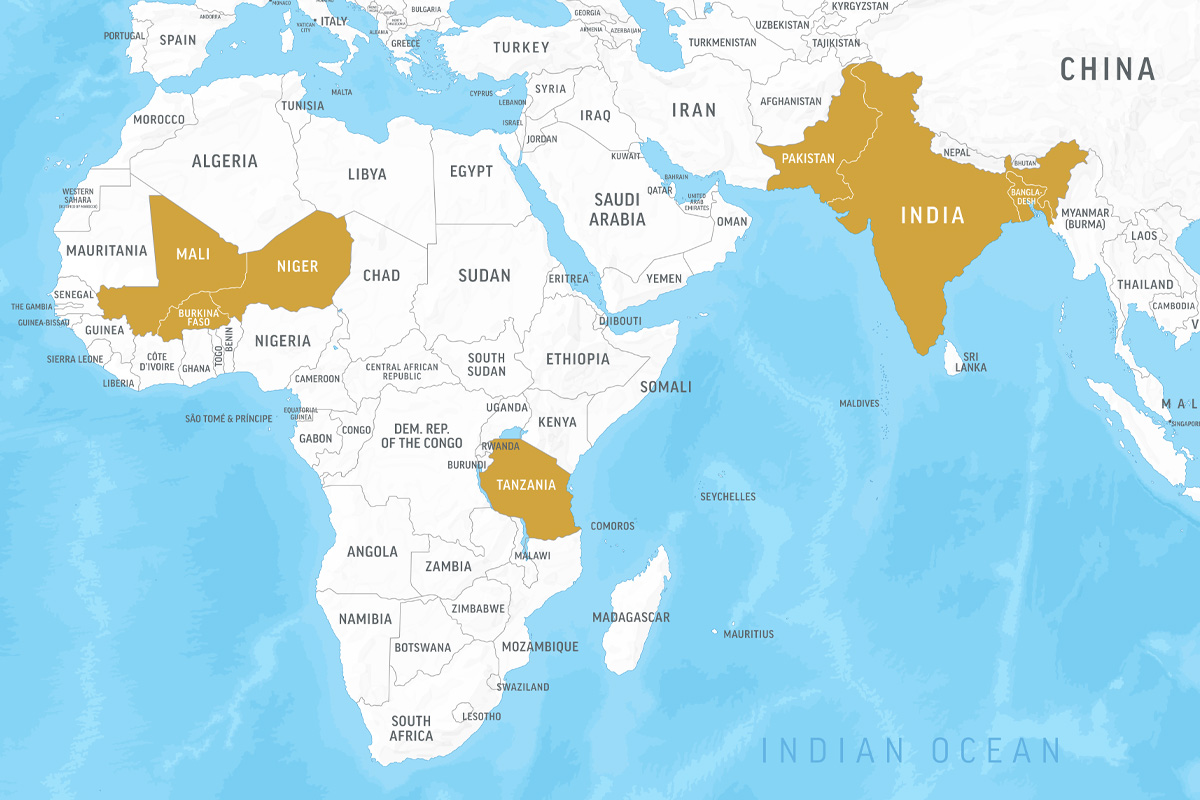
Ishita MostafaA mother and her child in Kurigram, Bangladesh, participated in a clinical trial of a microbiome-directed food designed to nurture beneficial gut microbes and treat childhood malnutrition. The supplementary food — developed at Washington University School of Medicine in St. Louis — is now being evaluated in major randomized controlled trials funded by the Bill & Melinda Gates Foundation and carried out in collaboration with the World Health Organization (WHO) and UNICEF. The trials will involve nearly 20,000 children in Bangladesh, India, Pakistan, Mali, Tanzania, Burkina Faso and Niger.
A staggering 3 million children die from malnutrition across the globe each year, with many more left with long-lasting deficits in their growth and development. Jeffrey I. Gordon, MD – widely regarded as the father of the microbiome – has dedicated his life’s work to changing this paradigm. Gordon, of Washington University School of Medicine in St. Louis — along with WashU colleague Michael J. Barratt, PhD, a team of collaborators led by Tahmeed Ahmed MBBS, PhD, at the International Centre for Diarrhoeal Disease Research, Bangladesh (icddr,b), and a host of graduate students, postdoctoral researchers and staff scientists in Gordon’s lab — have developed a treatment for childhood malnutrition that nurtures the beneficial microbes in the gut, with the aim of more effectively treating the condition.
After success in early clinical trials led by Ahmed in Bangladesh, the innovative “microbiome-directed” food the team has developed will be evaluated in major randomized controlled trials funded by the Bill & Melinda Gates Foundation and carried out in collaboration with the World Health Organization (WHO) and the United Nations Children’s Fund (UNICEF). The trials will involve nearly 20,000 children in seven countries in South Asia and Africa: Bangladesh, India, Pakistan, Mali, Tanzania, Burkina Faso and Niger.
 Getty Images/Beth Anglin
Getty Images/Beth AnglinEarlier studies have shown that this treatment — a supplementary food with soybeans, chickpeas, peanuts and bananas as key ingredients — improves weight and height recovery in malnourished children more effectively than a traditional nutrient-dense, high-calorie food typically used to treat malnutrition. Gordon’s work has shown that beneficial strains of gut bacteria that support growth and development in infants and children thrive on specific biochemical components in these foods.
In addition, the team found that malnourished children receiving the microbiome-directed therapeutic food in earlier trials had higher levels of blood proteins involved in neurodevelopment and musculoskeletal growth, and lower levels of inflammatory proteins than children receiving a traditional nutritional intervention for malnutrition. Together, these results suggest that the effects of microbiome repair through food formulation extend far beyond the walls of the gut.
 Matt Miller
Matt Miller“Far too many children are still dying of malnutrition, and those who survive face lifelong consequences — stunted growth, neurological problems and immune system deficiencies, among others,” said Gordon, the Dr. Robert J. Glaser Distinguished University Professor and director of the Edison Family Center for Genome Sciences & Systems Biology. “These children are deprived of the ability to reach their full potential because of the devastating effects of malnutrition. There is an enormous need for better treatments that we hope to address.”
Newman gift establishes Childhood Malnutrition Program
Even as the clinical trials get underway, Gordon is focused on understanding the complex roles of the gut microbiome in the health of infants and children. With support from a recent gift from Andy Newman, vice chair of Washington University’s Board of Trustees, Gordon established the Newman Initiative for Childhood Malnutrition, now called the Child Undernutrition Program (CUP), which is co-led by Barratt, an associate professor of pathology & immunology. The gift builds upon a 2017 gift from the Harry Edison Foundation that established the Edison Family Center for Genome Sciences and Systems Biology, which Gordon leads. Newman is a nephew of Harry Edison.
“Jeff Gordon’s pioneering focus on the microbiome has importantly become the science likely to improve the lives of millions of children suffering from malnutrition,” Newman said. “It’s an honor to support the Gordon Lab at Washington University in this transformative work. Anything that we can all do to accelerate the use of microbiome-directed foods by the children of the world is welcome.”
Added Gordon: “The Child Undernutrition Program is a catalyst to advance the field as rapidly as possible and to ensure that there are timely investments in technology, knowledge building, implementing new discoveries, and education as we train the next generation of investigators,” Gordon said. “We are grateful to Andy Newman for his ongoing support, which is fueling critical discoveries that will help shape the future of the field and have the potential to improve the outlook for millions of children suffering from malnutrition around the globe.”
“We also want to keep in mind our long-term goal, which is that the lessons we are learning about microbiome health and nutrition — through new clinical trials that span a number of low- and middle-income countries in Africa and South Asia — are applied to our own communities in the U.S., including St. Louis,” Gordon said.
Gordon, Barratt and their colleagues, as part of the clinical trials, will perform detailed analyses of blood and stool samples from some of the trial participants to determine the effects of the foods on the gut microbiomes of the children, as well as the effects that gut microbes and diet treatment have throughout the body, indicated by blood biomarkers of immune function, metabolism, bone formation and neurologic development. These features will be assessed in conjunction with various clinical measurements of growth and body composition performed at the study sites.
Healthy development of gut microbial community essential early in childhood
Gordon’s work has shown that the formation of a healthy gut microbial community in the first two years of life is a central facet of healthy growth and development in children. Furthermore, Gordon said there is a critical window in early childhood when interventions with beneficial foods could help shape the development of the gut microbiome in a way that promotes healthy growth.
“We rely on our gut microbes to help us metabolize beneficial dietary components that we can’t process on our own and that are essential to good health,” Gordon said. “When a child’s gut microbiome is underdeveloped, their overall growth can stagnate, leading to malnutrition with further detrimental effects on immunity, metabolism, bone growth and brain development. Our previous studies indicate that certain foods can help repair underdeveloped microbiomes, and we’re hopeful these clinical trials will give us new insights into how best to nourish these children to restore their healthy growth and development.”
In children under age 5, malnutrition is a leading cause of death globally, with stunting affecting almost 150 million children from birth to age 5 worldwide, according to the World Health Organization and UNICEF. While treatment with traditional nutrient-dense foods reduces overall mortality among malnourished children, their impact on the lingering effects of malnutrition has been limited.
Microbiome-directed therapeutic food to be evaluated in nearly 20,000 children
The new trials of the microbiome-directed food in Burkina Faso and Niger each will include about 6,500 children ages 6 months to 2 years who are either moderately or severely malnourished. Children will be randomly assigned to receive either a standard supplementary food or the microbiome-directed food to supplement their normal diets.
The trials in Bangladesh, India, Pakistan, Mali and Tanzania each will include about 1,270 children (about 6,300 total across all five sites) ages 6 months to 2 years with moderate acute malnutrition. As part of the trial, these malnourished children also will have some type of illness, such as malaria, diarrhea, pneumonia or fever, which dramatically increases their risk of death. The researchers are investigating whether the microbiome-directed food can not only improve weight gain in these children, but also help them recover from illness more quickly than if treated with traditional supplementary foods, or with locally available foods typical of the diet of that community.
All of the trials will include comparisons to groups of children with healthy growth patterns, from the same communities, who are followed from 6 months to 2 years of age, with regular collection of stool and blood samples for analyses of the microbiome and the same blood biomarkers as performed for the malnourished children. These children will eat their normal diets, which will not include the supplementary foods.
If the microbiome-directed food is found to be superior to current therapies in helping malnourished children recover from infections and show improvements in various features of impaired growth that is characteristic of malnutrition, the trials could lead to a change in WHO policy on how undernutrition in infants and children is treated.
Gordon and his colleagues also are seeking a thorough understanding of the specific microbes that process these foods. Such knowledge eventually may lead to therapies that provide specific microbial strains in combination with the foods that help the beneficial microbes thrive. Most recently, Gordon and his team have defined important biochemical components of the microbiome-directed food and the key bacterial strains that process these components.
Team’s research continues to elucidate role of gut microbiome
Reporting recently in Nature and Nature Microbiology, the researchers found that the microbiome-directed food contains specific types of polysaccharides, including galactans and mannans, that are less abundant in traditional therapeutic foods for malnutrition. Their recent clinical and preclinical studies have identified strains of Bifidobacterium infantis and Prevotella copri in the gut microbial communities of children; these are important mediators of healthy growth. Moreover, certain strains of P. copri were found to be the principal consumers of the galactans and mannans in the microbiome-directed food. Experiments involving germ-free mice fed the microbiome-directed food and colonized with specific strains of P. copri recovered from stool samples from clinical trial participants demonstrated the interaction between the microbiome-directed food and P. copri plays a causal role in promoting healthy growth and metabolism.
Throughout his career, Gordon has trained 145 graduate students and postdoctoral researchers who have contributed to the foundational research that led to the new trials. A universitywide symposium April 25 and 26 in the Eric P. Newman Education Center on the Medical Campus will focus on the gut microbiome and its influence on health and disease. The symposium, titled “Eras of the Microbiome,” will celebrate the careers of researchers who have trained in Gordon’s lab, a number of whom have become leaders in the field of microbiome research. Twenty of his trainees will give lectures about their current microbiome work.
“This is a very exciting time for the field,” Gordon said. “We look forward to learning from these clinical trials of microbiome-directed foods for childhood malnutrition about how components of our gut microbial communities shape different facets of healthy growth. We are also deeply invested in nurturing our collaborations with international colleagues so that together we can build the capacity for clinical microbiome programs in low- and middle-income countries focused on infants, children and their mothers, and implement, at a societal level, the therapeutic and preventive strategies that are being developed as a result of this work. We are thrilled that these efforts are occurring at time when Washington University is establishing a new School of Public Health.”







