Immune modulator drugs for COVID-19 focus of major NIH clinical trial
Phase 3 trial involves hospitalized patients with moderate to severe disease
 Matt Miller
Matt MillerWilliam G. Powderly, MD, discusses patient care with Maanasi Samant, MD, in the intensive care unit at Barnes-Jewish Hospital. Powderly, director of the Institute of Clinical and Translational Sciences at Washington University School of Medicine in St. Louis, is protocol chair of an international phase 3 clinical trial funded by the National Institutes of Health (NIH) to investigate the potential of three drugs for the treatment of COVID-19. The drugs were chosen for their potential to tame a dangerous inflammatory response experienced by some patients with the virus.
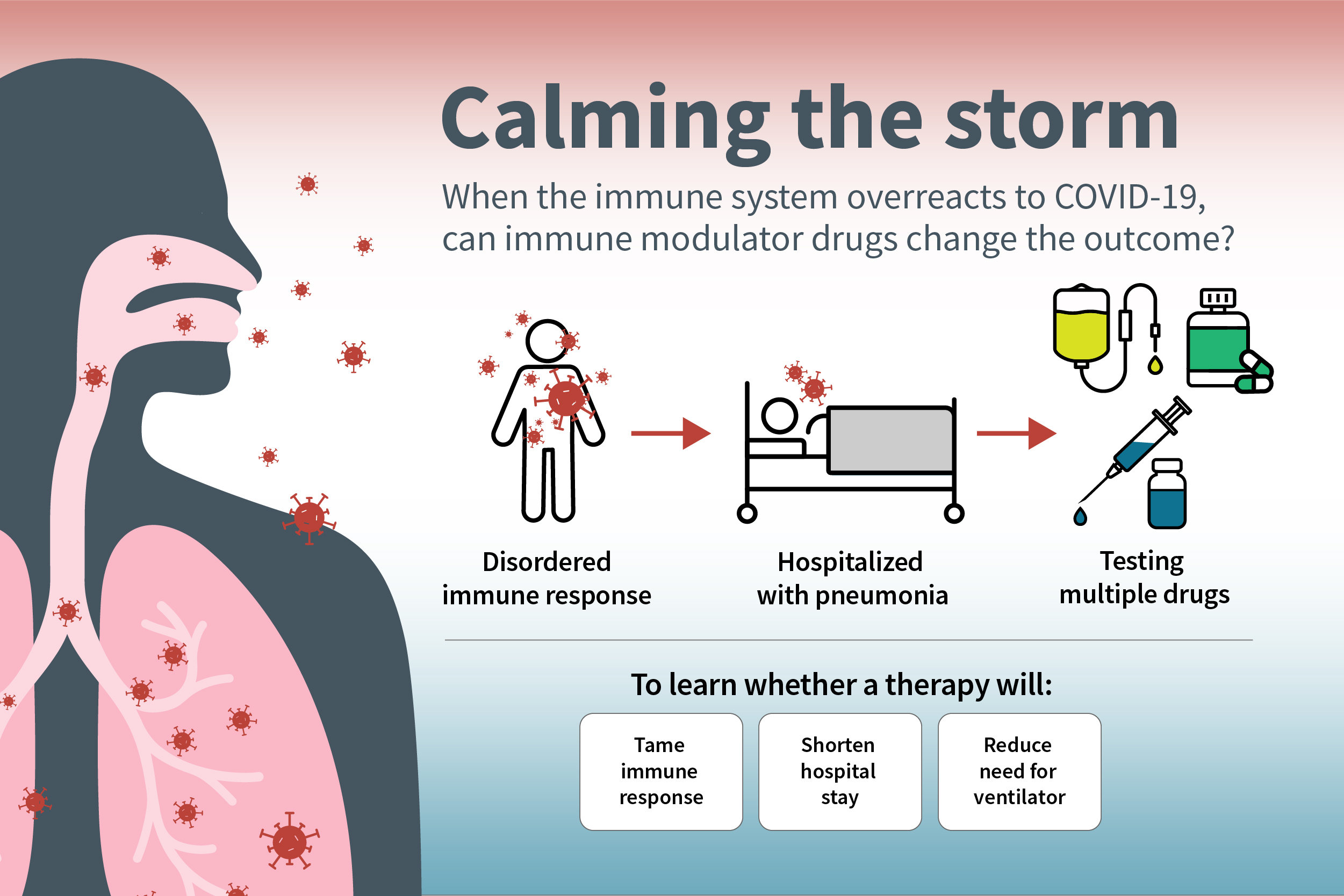
One of the most vexing aspects of the COVID-19 pandemic is the novel coronavirus’s ability to turn the body’s immune system against the body. After the virus has been cleared from the body, the immune system sometimes continues to hurtle an arsenal of immune proteins at the already vanquished virus, creating a dangerous inflammatory response called a cytokine storm. Such cytokine storms can contribute to acute respiratory distress syndrome, multiple organ failure and death.
A new, international phase 3 clinical trial, led by Washington University School of Medicine in St. Louis and funded by the National Institutes of Health (NIH), will investigate the potential of three drugs — developed for other inflammatory diseases — to tame the cytokine storm, shorten hospital stays and reduce the need for patients to be placed on ventilators to help with breathing.
The goal is to enroll 2,000 patients hospitalized with moderate to severe COVID-19 across the U.S. and Latin America. All patients will receive standard of care treatment, which includes remdesivir, an antiviral drug that has shown some ability to speed recovery and reduce mortality. In addition to standard therapy, the participants will be randomly assigned to receive a placebo or one of three anti-inflammatory drugs.
“In severe COVID-19 infection, we think the virus triggers an abnormal immune response, which drives inflammation in the lungs and is the major reason people end up needing ventilators and sometimes dying,” said Washington University’s William G. Powderly, MD, protocol chair of the international trial, the J. William Campbell Professor of Medicine, and director of the Institute of Clinical and Translational Sciences. “The viral infection can trigger the need for hospitalization, but the illness that we see in such patients is predominantly an aberrant immune response. About 10 days after the initial infection — around the time many people with severe illness are hospitalized — many don’t have the active virus anymore, so an antiviral drug can’t do much at this point. We think adding an immune modulator drug may be helpful, and this trial is designed to determine whether such drugs can benefit patients with severe illness.”
The trial, part of the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV) initiative, is being coordinated and overseen by the National Center for Advancing Translational Sciences (NCATS), part of NIH. It is expected to last about six months, and COVID-19 patients at Barnes-Jewish Hospital may be eligible to participate in the trial.
Results will be available shortly after the trial is completed or possibly sooner if an analysis conducted during the trial indicates that one of the drugs is beneficial. To ensure that the trial is being conducted safely and effectively, an independent data and safety monitoring board will oversee the trial and conduct periodic reviews of the data.
The drugs being evaluated in the trial are infliximab, under the trade name Remicade, developed by Janssen Research & Development LLC, one of the Janssen Pharmaceutical Companies of Johnson & Johnson; abatacept, developed by Bristol Myers Squibb under the trade name Orencia; and cenicriviroc, an investigational drug developed by AbbVie that is in late-stage clinical trials for inflammatory disorders.
All three are designed to curb the immune system and reduce inflammation. Infliximab is approved by the Food and Drug Administration (FDA) to treat several chronic inflammatory conditions such as rheumatoid arthritis, Crohn’s disease and ulcerative colitis. Abatacept is FDA-approved to treat various forms of arthritis, including rheumatoid arthritis, psoriatic arthritis and polyarticular juvenile idiopathic arthritis. Cenicriviroc is being evaluated in clinical trials for treatment of liver inflammation caused by a buildup of fat in the liver, and has been studied clinically for treatment of HIV infection.
The three immune modulator drugs were selected for the study from a list of over 130 medications known to regulate the immune system. They were chosen based on their likely relevance in treating COVID-19, evidence for use against inflammation and cytokine storm, safety profile and broad availability for large-scale clinical studies.
The clinical trial design is what is known as an adaptive master protocol. This allows the investigators to evaluate as many as five drugs at one time, and stop or start drug evaluations based on the current evidence. Drugs that don’t show promise can be eliminated from the study and replaced with others. The trial also allows the flexibility to change the standard of care if a separate trial identifies new therapies as safe and effective for patients enrolled in the ACTIV trial.
“If a drug is not working, we can rapidly take it out of the protocol, and if new therapies become available, they can be added,” said Powderly, also director of Washington University’s Institute for Public Health and co-director of the Division of Infectious Diseases at the School of Medicine. “All patients in the trial will receive the best standard of care therapy, which includes remdesivir, plus they will have a three out of four chance of receiving one of the investigational drugs.”
The Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV) initiative is a public-private partnership, with funding provided by Operation Warp Speed through the Biomedical Advanced Research and Development Authority of the U.S. Department of Health and Human Services’ Office of the Assistant Secretary for Preparedness and Response.
The NIH announced the ACTIV initiative in April. The goal of the program is to develop a national research response to prioritize and speed the development of the most promising COVID-19 treatments and vaccines. Led by the Foundation for the National Institutes of Health, ACTIV coordinates partnerships between government, industry, academia and nonprofit organizations.
 Sara Moser
Sara Moser







