A changing landscape: Alzheimer’s disease, research and the future of care
Ongoing research is changing the outlook on Alzheimer's from hopeless to hopeful
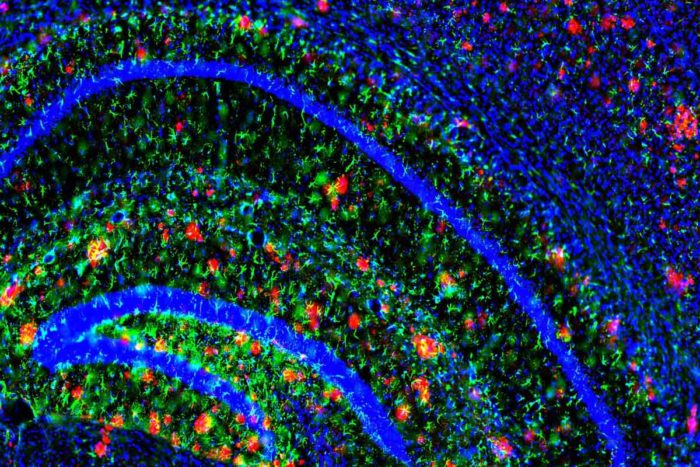 Image courtesy of John Cirrito, PhD
Image courtesy of John Cirrito, PhDAmyloid plaques (in red) dot the brain of a mouse model of Alzheimer’s disease. Mouse models have been critical in uncovering the molecular processes that contribute to Alzheimer’s.
Most of us have been touched by Alzheimer’s disease, whether through a parent or grandparent, a husband or wife, or a friend caring for a loved one. Apart from the devastating symptoms of the disease, what frustrates many of us is how little can be done to help those living with the disease. Yes, there are medications available that can modestly and temporarily slow progression of Alzheimer’s for some, but there are no clinical solutions for stopping it.
Ongoing research, however, is changing the landscape from hopeless to hopeful.
Building on the research that began several decades ago, scientists studying Alzheimer’s—many of them at Washington University School of Medicine—are developing a progressively clearer picture of what causes the disease. David Holtzman, MD, Barnes-Jewish Hospital’s neurologist-in-chief and the chair of neurology at Washington University, notes that the university’s community of Alzheimer’s researchers is making significant contributions to the field by studying people who are living with the disease.
Holtzman says, “Working with advanced technologies and studying people at various stages of cognitive health, we’ve identified changes in the brain associated with Alzheimer’s and learned a great deal about how Alzheimer’s progresses. Although there are no cures yet, we are studying promising treatments.”
A brief history
Auguste Deter, a German woman living in the early 20th century, was reported by her physician, Alois Alzheimer, to exhibit a cluster of unusual symptoms considered evidence of mental illness: memory loss, language difficulties and unpredictable behavior. Alzheimer examined Deter’s brain after her death and found it to have unusual tangles of fibers and abnormal clumps. A few years later, in 1910, Emil Kraepelin, a psychiatrist and colleague of Alzheimer’s, published a medical book in which he described Auguste Deter’s symptoms and the results of her autopsy. Kraepelin coined the phrase “Alzheimer’s disease” to name her disorder.
But it wasn’t until the 1970s that Alzheimer’s disease began to be studied in earnest in the United States, in part as a response to knowledge that the country’s population was rapidly aging. In 1974, Congress established the National Institute on Aging (NIA), making it part of the National Institutes of Health (NIH).
Building blocks
In 1979, Washington University neurologist and scientist Leonard Berg, MD, began his studies of the disease. Berg’s strategy was to take a collaborative approach, gathering colleagues who were nationally known specialists in the fields of neurology, imaging, pathology, psychiatry and psychology, among other disciplines—all on the faculty at Washington University. The resulting team had the expertise to tackle Alzheimer’s from multiple angles.
Ultimately, Berg, now deceased, and his team developed a diagnostic tool called the clinical dementia rating scale. Considered the worldwide standard for assessing dementia in patients with Alzheimer’s, the tool allows a practitioner to rate a patient on a range from 0 (no dementia) to 3 (severe dementia) using a series of interviews.
Then in 1985, Berg founded the Charles F. and Joanne Knight Alzheimer’s Disease Research Center (Knight ADRC) at Washington University. Since Berg’s time, the center’s scope has mushroomed to include approximately 40 faculty members from 15 Washington University departments—a collection of basic scientists and physicians who treat patients at Barnes-Jewish Hospital—along with many collaborators worldwide.
Neurologist John Morris, MD, who now directs the center Berg founded, says, “The rating scale developed at Washington University continues to be a very reliable way to assess a patient’s degree of dementia and gauge changes over time. It’s an important tool in patient care and research.” Equipped with a way to measure Alzheimer’s progression and a center to facilitate efficient study of the disease, researchers at Washington University and elsewhere had a foundation upon which they could build multiple lines of inquiry.
Targeted research, new discoveries
To gain a precise understanding of Alzheimer’s, scientists needed to understand the role genes play in the disease. Genes orchestrate the production of proteins and other substances that carry out the body’s functions at a molecular level. When research connects a gene flaw or characteristic to a disease, the identified genes are considered potential troublemakers and can become targets of study themselves.
Alzheimer’s and genetics
In the early 1990s, researcher Alison Goate, DPhil, identified the first genetic mutation for an inherited form of early-onset Alzheimer’s disease, in which symptoms begin before age 60.
At Washington University, Goate and a group of international collaborators also identified several more gene mutations that predispose people to develop Alzheimer’s, both early- and late-onset. These genes direct the production of a protein called amyloid beta, the main ingredient in Alzheimer’s brain plaques, and another protein called tau, a component of Alzheimer’s tangles. Her work pointed to these substances as playing a critical role in forming the tangles and clumps Alois Alzheimer found when he studied his patient’s brain tissue. Goate now directs the Ronald M. Loeb Center for Alzheimer’s Disease at the Icahn School of Medicine at Mount Sinai in New York.
According to Morris, one significance of Goate’s findings is this: “We can now use genetic analysis to help identify people who are at high risk for Alzheimer’s. And we’re working to understand which forms of genetic proteins are involved and define exactly what goes wrong.”
At the molecular level
In the labs at Washington University, Holtzman and colleagues have made significant progress in understanding the molecular processes that allow plaques and tangles to form. Specifically, they’re investigating what mechanisms regulate the body’s levels of amyloid beta and tau, among other substances. The results of their work may lead to the development of drugs to correct those disease mechanisms and reduce or avoid the buildup that causes Alzheimer’s brain plaques and tangles.
In 2006, Holtzman and fellow neurologist Randall Bateman, MD, developed a technique called SILK (stable isotope linked kinetics) that monitors how quickly amyloid beta is produced in and cleared from the central nervous system. Using this powerful tool, the researchers learned that the overabundance of amyloid beta in people with Alzheimer’s isn’t caused by an overproduction of that protein. Instead, amyloid beta accumulates because the body has a reduced ability to rid the substance from the brain.
Holtzman and Bateman have also learned that amyloid-beta clearance slows down dramatically with age; as clearance rates go down, brain levels of amyloid beta rise, increasing the chances that the protein will form plaques. Studying mice, Holtzman has found that elevated blood sugar can increase amyloid-beta levels—information that may explain why there is a link in humans between diabetes and an elevated risk for Alzheimer’s disease.
An Alzheimer’s timeline emerges
Another key to understanding and treating Alzheimer’s is knowing when it begins. Since the early 1990s, Washington University researchers have been collecting evidence that suggests the disease causes brain damage well before symptoms of dementia appear.
Among that evidence: Autopsy studies by Morris and neuroanatomist Joseph Price, PhD, DPhil, showed that 30 percent of cognitively normal people age 65 and older have Alzheimer’s plaques. And beginning in 2004, Morris and colleagues have used radiology to produce images of amyloid-beta plaques in people who are living with the disease. For many years, the only reliable way to confirm the presence of Alzheimer’s plaques was after death, through a brain autopsy.
Initially, Morris and Price studied individuals who showed Alzheimer’s symptoms; later, they published the first-ever report showing that brain plaques develop silently in some people who don’t have cognitive deficits and that the plaques are associated with an increased risk of developing dementia.
Research by Yvette Sheline, MD; Beau Ances, PhD, MD; and others takes this finding a step further: Using functional magnetic resonance imaging, or fMRI, they have shown that brain function declines even before plaques appear.
“We currently believe that, from the time excess amyloid begins to collect in the brain, it takes about 10 years for a person to develop dementia,” says Sheline, who is now at the University of Pennsylvania. “Our studies suggest we might be able to intervene before plaques begin to form. If effective treatments are developed, we might have an even bigger window of time to intervene.”
Sleep and Alzheimer’s
Sleep—or, more accurately, the lack of it—is beginning to look like an early indicator of Alzheimer’s. Holtzman and colleagues were among the first to connect early stages of Alzheimer’s to sleep disruption in humans. In 2009, they found that amyloid beta is regulated by the sleep-wake cycle and that sleep deprivation accelerates amyloid-plaque development.
The same team later found that sleep is disrupted in people who likely have early Alzheimer’s disease but don’t yet show symptoms and that sleep loss contributes to the growth of plaques and increases the risk of dementia. Studying mice, the research team found that a sleep-regulating protein called orexin is involved; when orexin production is blocked, mice sleep better and plaque production slows down.
“As we start to treat people who have markers of early-stage Alzheimer’s, changes in their sleep patterns may help us determine whether treatments are working,” Holtzman says. “And we should be looking hard at orexin as a potential target for preventing Alzheimer’s.”
For the curious
For more information about Alzheimer’s disease, visit the following websites:
National resources:
At Washington University:
The big questions: Who will get Alzheimer’s—and when?
Because researchers now know that Alzheimer’s damage starts with a silent phase years before symptoms appear, the goals for treatment have shifted. “We want to prevent damage and loss of brain cells by treating the disease early, before outward symptoms are evident,” says Bateman. “If we wait until symptoms begin, it may be too late.”
But to make preventive treatments practical for late-onset Alzheimer’s—the more common form of the disease associated with aging—researchers and clinicians need accurate ways to determine which individuals should receive treatment and when. “There are risks in Alzheimer’s treatments, so we have to be careful that the people we treat to prevent dementia really are destined to develop the disease,” Morris says.
Biomarkers are a promising solution to this problem. Monitoring these substances—which are naturally produced by the body—can help to identify the onset of the disease and then track its progression. Since 2006, Anne Fagan, PhD, and others at Washington University have found several biomarkers in the cerebrospinal fluid, which circulates in the brain and spinal cord. Studying patients with Alzheimer’s, these researchers have shown that monitoring the levels of certain biomarkers, including amyloid beta and tau protein, is a reliable way to identify the presence of the disease up to 20 years before symptoms appear. And the biomarkers can be monitored to track progression of the disease.
Applying these biomarkers and radiology tests, Bateman and colleagues have developed a detailed timeline of physical changes in the brain, with the first sign of disease showing up 25 years before symptoms appear. Because their work required participants who had an extremely rare inherited type of Alzheimer’s, the research team established an international research consortium of 13 institutions to recruit enough patients. Called the Dominantly Inherited Alzheimer Network (DIAN), the consortium is led by Bateman and colleagues at Washington University
In collaboration with investigators at the University of Maastricht in the Netherlands, the Washington University team has now validated a new system that uses biomarkers to identify and formally classify individuals in the pre-symptomatic stages.
“We will likely assess the success or failure of Alzheimer’s treatments by monitoring changes in the levels of these biomarkers,” Fagan says. She notes that there is more work to be done in this area of research, “but results so far are compelling and encouraging.”
Treatment begins
The long and hard-fought campaign for solutions has reached a significant milestone: the launch in 2013 of the first clinical trial of anti-amyloid drugs designed to prevent Alzheimer’s dementia.
The trial is being conducted by the DIAN Trials Unit (DIAN-TU) and involves the same research teams and trial participants that produced Bateman’s biomarker timeline. Bateman directs the DIAN-TU and is principal investigator of the international trial.
Currently, the trial is studying two drugs: gantenerumab and solanezumab, both aimed at countering the toxic effects of amyloid beta. Solanezumab was developed by Eli Lilly and Company in collaboration with Holtzman. Future plans call for evaluating additional drugs and for other institutions to participate in the trial.
The trial involves approximately 138 people who have inherited mutations for early-onset Alzheimer’s and either have no symptoms or have mild symptoms, and roughly 27 family members who don’t have these mutations. Researchers are tracking their brain health to see whether disease progression strays from the pattern already documented in their relatives with active Alzheimer’s. The investigators hope to apply what they learn to develop treatments for the more common forms of Alzheimer’s.
 Robert Boston
Robert Boston“We’ve reached a very exciting moment in Alzheimer’s disease research, and it gives me renewed hope for a future without Alzheimer’s,” says Brent Whitney, a participant in the trial. “I hope my grandchildren someday learn of this condition in history books, like I learned about polio.”
In the meantime, researchers are also studying people known to be in the very early stages of Alzheimer’s disease, before symptoms of memory loss and dementia are apparent; they are looking for ways to determine when cognitive decline begins. And their goal is to determine the optimal time to begin preventive treatments.
“We want to find a signal that will tell us that a particular person is on the path to progress from cognitive normality to symptomatic Alzheimer’s disease,” Morris says, “because that would identify the perfect time for us to begin treatment.”







