Cancer stem cells drive brain tumor growth
Targeting cancer stem cells, which make certain cancers resistant to treatment, could improve outcomes for patients
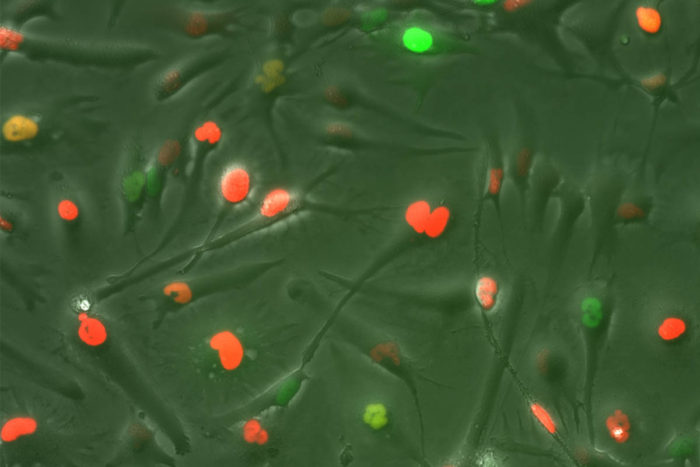
Cancer stem cell line from a patient with glioblastoma. The red, green and yellow colors indicate different phases of the cell cycle.
Researchers at Washington University are studying a type of tumor-generating cell thought to be responsible for certain forms of treatment-resistant brain cancers. Developing therapies targeting these cells holds promise for improving survival and combating cancer relapse.
The cells are popularly referred to as cancer stem cells, or tumor-initiating cells (TICs). Like embryonic stem cells, they regenerate and give rise to cells that mature into various cell lineages. But unlike the embryonic version, TICs generate uncontrolled growth of cancer cells, eventually forming a tumor made up of multiple types of cancer cells.
Although radiation and chemotherapy can kill tumor cells generated from TICs, these therapies often do not kill the TICs themselves. Studies have shown that if TICs are present and aren’t destroyed, tumors recur.
“It’s almost like they are zombie cells,” says Albert Kim, MD, PhD, a Washington University neurosurgeon. “We don’t have any good therapies to combat them, so we need to think of new strategies.”
Targeting aggressive brain cancer
Kim studies TICs in glioblastoma, a common and particularly aggressive type of brain cancer. One theory on how to kill TICs is to try to force them to change into a particular, non-cancerous cell lineage, in other words, to differentiate them. Researchers who have done this in cell culture find that differentiated TICs don’t form tumors as effectively in animal models.

TICs tend to grow in certain “microenvironments,” such as areas with an increased number of blood vessels, or abnormally low glucose or oxygen levels, says Kim. “Another way to get rid of TICs may be to disrupt these microenvironments so that TICs can no longer survive there,” he says.
Kim is involved in a third approach: targeting TICs using disulfiram, commonly known as Antabuse, an FDA-approved drug sometimes given to alcoholics to prompt an adverse reaction if they consume alcohol. Researchers found that in a drug screen, disulfiram killed TICs in certain brain tumors. The drug disrupts protein regulation in TICs, leading to toxicity and cell death.
With Kim’s help, radiation oncologist Jiayi Huang, MD, is running a clinical trial to test the safety and efficacy of disulfiram in glioblastoma patients who have already undergone radiation therapy. Their hope is that the drug may improve patients’ clinical outcomes.
Targeting TICs may be a key way to control the spread of treatment-resistant cancers in the brain and elsewhere. “If we could make deadly cancers like glioblastoma into a chronic, treatable disease, that would be a huge triumph,” says Kim.







