Boosting T cells improves survival in mice with glioblastoma
Treatment with interleukin-7 revs up immune system against deadly brain tumor
 Washington University School of Medicine
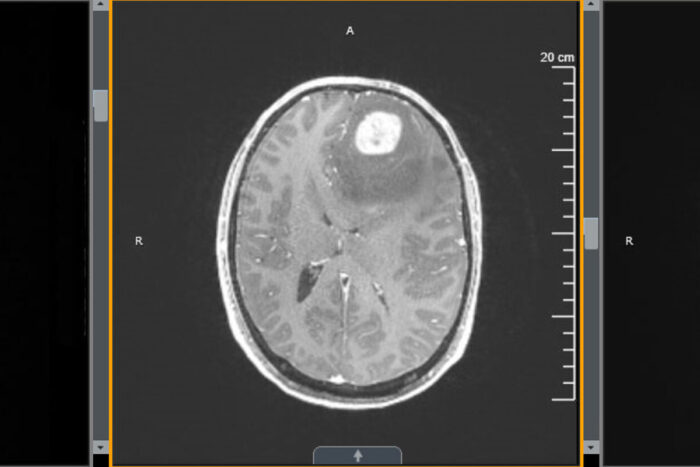
Washington University School of MedicineA new study from Washington University School of Medicine in St. Louis shows that treatment with an immune-boosting protein called interleukin 7 (IL-7) in combination with radiation improves survival in mice with glioblastoma. Shown is an MRI scan of the brain of a patient with glioblastoma.
Glioblastoma, an aggressive cancer in the brain or spinal cord, has proven stubbornly resistant to newer immunotherapies. And radiation and chemotherapy, the standard treatment for glioblastoma, result in fewer than 10% of patients surviving longer than five years after diagnosis.
But a new study from researchers at Washington University School of Medicine in St. Louis shows that treatment with an immune-boosting protein called interleukin 7 (IL-7) in combination with radiation improves survival in mice with glioblastoma. The new mouse study shows that IL-7 increases the number of T cells in the tumor and other immune organs. Such immune cells can then attack the cancer cells and improve survival.
The findings are published Jan. 14 in Clinical Cancer Research, a journal of the American Association for Cancer Research.
The study in mice suggests promise for a phase 1/2 clinical trial at Siteman Cancer Center at Barnes-Jewish Hospital and Washington University School of Medicine in St. Louis that is investigating a long-acting type of IL-7 in patients with glioblastoma.
Radiation in combination with chemotherapy is the standard of care for various cancers including glioblastoma. Although beneficial against cancer, these treatments also can impair patients’ T cells, known as lymphocytes, that are important for fighting infections. Many glioblastoma patients have low levels of T cells. Glioblastoma patients who have chronically low lymphocyte counts don’t survive as long as patients with higher numbers of these T cells.
“Previously, a multicenter study from the American Brain Tumor Consortium showed a six-month shorter survival for patients with low versus normal numbers of T cells,” said first author Jian L. Campian, MD, PhD, who conducted the research at Washington University School of Medicine and the Brain Tumor Center at Siteman. “We knew that glioblastoma patients with low lymphocytes surprisingly also have low IL-7, which is a growth factor that supports T cells. Normally, people with low T cells should have a high level of IL-7. We wanted to find out if giving IL-7 to patients could increase the numbers of T cells and, in the process, have a positive impact on survival.”
The researchers found that mice with glioblastoma tumors treated with a combination of chemotherapy, radiation and IL-7 lived longer than mice that received only chemotherapy and radiation. On average, control mice that received no treatment lived about 20 days after tumor implantation. The mice that received IL-7 alone lived about 30 days, and those that received radiation alone lived about 35 to 40 days. The mice that received a combination of radiation and IL-7 lived at least 40 days, and many were still alive at 90 days. The longest survival was in the mice that received the triple combination of chemotherapy, radiation and IL-7, most of which lived at least 45 days, with many still alive at the 90-day mark.
“It’s difficult to know how these increases in survival in mice might translate to people,” said co-senior author Milan Chheda, MD, an associate professor of medicine. “If many of these mice are surviving at least three months by adding IL-7, we’re hoping to see some type of improvement in our patients who are treated with IL-7. As a basis for comparison, the chemotherapy given for glioblastoma is called temozolomide, and it was first approved because it improved patient survival by an average of slightly over two months.”
In addition to increasing T cell numbers in the tumor and the tumor’s environment, IL-7 treatment increased T cells in the blood and immune organs, including the thymus, spleen and lymph nodes, the investigators found. The therapy also reduced T regulatory cells, which are known to suppress the immune system in the microenvironment of brain tumors.
“We are encouraged by the results we are seeing in the mice,” said senior author Dinesh Thotala, PhD, an associate professor of radiation oncology. “We also see evidence that IL-7 could be considered as a replacement for temozolomide, especially among the nearly 70% of patients who have a type of tumor that does not respond well to this chemotherapy.”
The researchers explained that current immunotherapies called immune checkpoint inhibitors work by taking the brakes off immune cells that are already present. Since so many glioblastoma patients have depleted T cell numbers, it is perhaps not surprising that immune checkpoint inhibitors have not proven effective.
“If we are able to increase the number of T cells by giving IL-7, we would like to find out if adding immune checkpoint inhibitors would then increase T cell activity against the cancer cells,” said Chheda, who treats patients at Siteman Cancer Center.
Campian, who is now with the Mayo Clinic, said the researchers have plans to launch a follow-up study in glioblastoma patients at Washington University and the Mayo Clinic to determine whether combining immune checkpoint inhibitors with long-acting IL-7 boosts survival.







