Pancreatic cancer vaccine evaluated
New research is evaluating the effectiveness of a pancreatic cancer vaccine post surgery
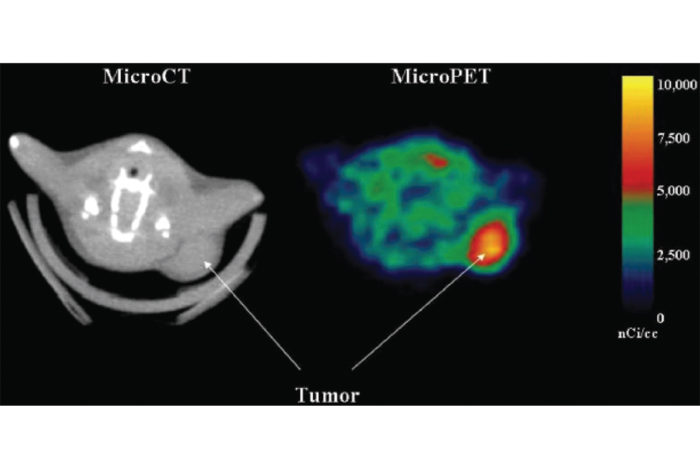
Pancreatic tumor imaging scans
A phase III clinical trial at Washington University School of Medicine and Barnes-Jewish Hospital is evaluating whether a vaccine developed to target pancreatic cancer cells can help prevent recurrences in patients who have had their pancreatic tumors surgically removed.
Nationwide, an estimated 700 patients with stage I/II pancreatic cancer will be enrolled in the trial. Half will receive the standard therapy, the chemotherapy drug Gemcitabine alone or with another chemotherapy drug, Fluorouracil (5-FU). The other half will receive the standard therapy plus the new pancreatic cancer vaccine.
The vaccine is designed to stimulate a patient’s immune system to recognize cancer cells as foreign invaders. It is composed of human pancreatic cancer cells exposed to radiation that have been genetically modified by inserting a mouse gene. The gene produces modified sugars not found in humans that are displayed on the surface of the cancer cells.
These sugars have the potential to stimulate a powerful immune response by the body that destroys the tumor cells or blocks their growth.
A new strategy for early-stage patients

“Few treatment options exist for patients with pancreatic cancer,’’ says William Hawkins, MD, a pancreatic surgeon at Barnes-Jewish Hospital who is leading the trial at Washington University. “This vaccine can potentially offer a new strategy for patients with early-stage cancer who have had surgery.”
These patients typically face a high rate of recurrence because stray cancer cells are often left behind, which can develop into another tumor.
Patients receiving the vaccine will receive a series of monthly injections beginning eight to 10 weeks after surgery. Afterward, they will be monitored every three months for three years, and then every six months for two more years. The researchers will determine whether the vaccine helps to reduce the rate of cancer recurrence and improve survival.
Data from an earlier phase II study of the same vaccine showed promising results. At 12 months after surgery, 91 percent of individuals in the trial survived. By 24 months after surgery, survival rates were 54. This improves upon the current median survival rate of 16 months. The researchers are continuing to follow and assess long-term survival benefits for patients in that trial.