Making aortic valve replacement an option for the inoperable
A procedure to replace a diseased heart valve has been approved for patients too frail to undergo open-heart surgery
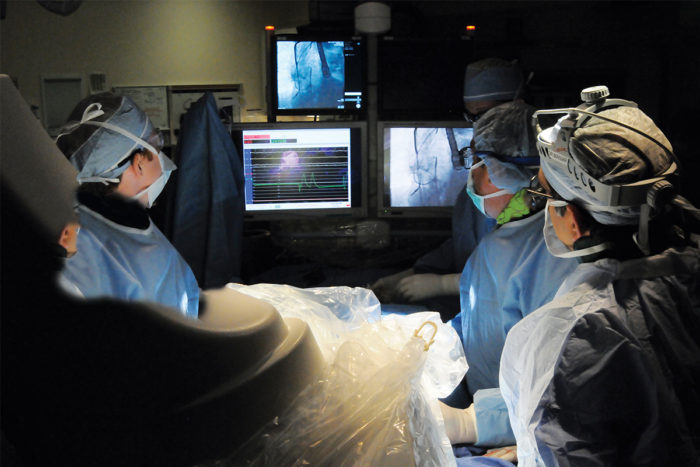
Left to right: Alan Zajarias, MD, John Lasala, MD, PhD and Hersh Maniar, MD, perform an aortic valve replacement.
Approval by the US Food and Drug Administration (FDA) of a procedure described as a “game changer” by physicians—transcatheter aortic valve replacement—now allows Washington University cardiac specialists at Barnes-Jewish Hospital to perform open-heart surgery without the “open” for patients previously unable to have surgery.
In the procedure, physicians are for the first time able to replace aortic valves less invasively. Instead of opening a patient’s chest, physicians thread a catheter, mounted with a compressed replacement valve on a tiny balloon, through an incision in an artery in the groin. Once in position, a stent-like valve is inflated in the aorta and the balloon and catheter are withdrawn.
PARTNER trial
The Washington University and Barnes-Jewish Heart & Vascular Center was one of only 23 centers nationally to participate in the nationwide PARTNER (Placement of AoRTic traNscathetER valves) trial leading to the FDA’s approval.
“It’s an effective way of treatment for patients who otherwise would remain untreated,” says Alan Zajarias, MD, Washington University interventional cardiologist at Barnes-Jewish.
“This is a phenomenal thing for patients who were not able to do their daily routine due to aortic stenosis. If they were not surgical candidates previously, we can now do something to help them live longer and feel better.”
The device, called the SAPIEN valve, developed by Edwards Lifesciences, consists of a heart valve made of cow heart tissue attached to a collapsible mesh stent.
Ralph J. Damiano Jr., MD, chief of cardiac surgery at the School of Medicine served with John Lasala, MD, PhD, as co-principal investigators of the PARTNER trial at the university. Other physicians conducting the trial are Hersh Maniar, MD, cardiac surgeon, Brian Lindman, MD, and Zajarias, who spent six months in France training with the procedure’s developer, Professor Alain Cribier, MD, chief of cardiology at the University Hospital in Rouen, France.
In an alternative procedure still under study, the investigational valve may also be inserted through an incision in the chest into the beating heart. This procedure is used for patients whose blood vessels are too small or contorted to accommodate the size of the catheter required.