Possible treatment strategy identified for bone marrow failure syndrome
Researchers working with pharma collaborations to develop better treatments
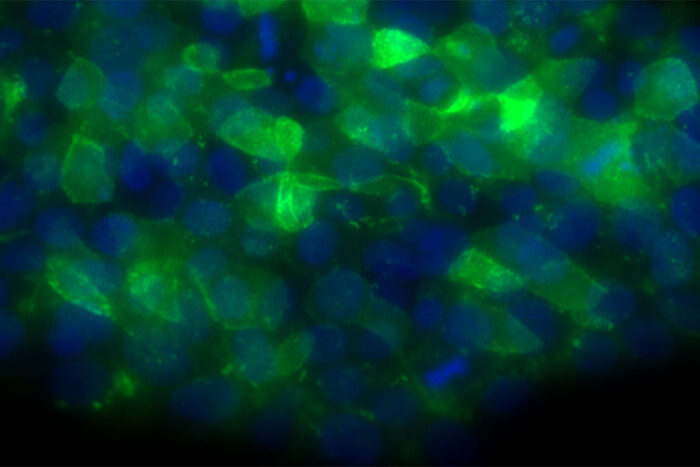 Batista Lab
Batista LabA new study from Washington University School of Medicine in St. Louis identifies a possible treatment strategy for some bone marrow failure syndromes. Shown are human embryonic stem cells engineered to have a mutation that causes poikiloderma with neutropenia, a bone marrow failure syndrome that increases a patient's risk of developing dangerous infections.
Bone marrow is the spongy tissue inside bone responsible for making red blood cells, white blood cells and platelets. Bone marrow failure syndromes lead to an increased risk of developing dangerous infections, anemia and an increased risk of blood cancers.
Research led by Washington University School of Medicine in St. Louis has identified a possible treatment strategy for a rare bone marrow failure syndrome that is named poikiloderma with neutropenia. The work also may have implications for treating other bone marrow failure syndromes with similar underlying dysfunctions.
The research is published March 3 in the journal Science.
Poikiloderma with neutropenia is caused by mutations in a gene called USB1. Despite knowing the genetic error that causes the disease, the specifics of what the error does to cause bone marrow failure have long been a mystery. When the bone marrow fails, the body can’t make healthy red blood cells, white blood cells and platelets. People with these types of diseases are at increased risk of infections and are prone to developing skin and blood cancers.
“There are no cures for poikiloderma with neutropenia,” said co-senior author Luis Batista, PhD, an associate professor of medicine. “Patients are at high risk of dying from complications of infections, and scientists had no idea why mutations in this gene lead to bone marrow failure. In this new study, we found a novel role for an enzyme that opens the door to future clinical trials. There are investigational drugs that block this enzyme, so we are hopeful that clinicians who treat these patients may find this a promising strategy to pursue.”
Studying human embryonic stem cells engineered to model this syndrome, the investigators, including co-senior author Roy Parker, PhD, of the University of Colorado, Boulder, found a problem with the processing of molecules called microRNA. The processing problem causes specific microRNA molecules to break down faster than they should. Without sufficient levels of these microRNAs, the stem cells can’t develop into normal blood cells.
“Our study shows that normal USB1 is cutting off the long tails of these microRNAs, which stabilizes their structure, giving them time to do their jobs forming blood products,” said first author Hochang Jeong, PhD, a postdoctoral research associate in Batista’s lab. “When USB1 is mutated in this disease, these microRNA tails are much longer than they should be. We know that having longer tails makes microRNAs and other classes of RNA molecules more easily targeted for degradation. What we learned is there should be an equilibrium between the enzyme that puts the tails on and the enzyme that chops off the tails.”
While there is not yet a known way to restore the ability to properly remove the tails, investigational drugs already exist that block the enzymes responsible for putting the tails on. Blocking this enzyme in this disease potentially could restore the equilibrium between the adding and subtracting of tails.
The enzymes responsible for adding the tails are called PAPD5 and PAPD7, and inhibitors of these enzymes have been investigated in human clinical trials for other diseases, including hepatitis B. For this study, the researchers used a PAPD5 inhibitor called RG7834. Preventing the addition of the long tail stabilized the structure of the microRNAs, increasing their levels and restoring normal blood cell formation by these stem cells. The researchers are working with industry partners to develop new PAPD5 and PAPD7 inhibitors that are specifically designed to treat this and similar conditions.
“We are working with different companies to develop better and more specific PAPD5 inhibitors to treat this rare syndrome,” Batista said. “In my lab, we are big advocates for the study of rare diseases. Combined, rare diseases are not rare at all, and these patients deserve our attention. PAPD5 inhibition is poised to be a potential treatment for other bone marrow failure syndromes.”